Data-processing statistics for P. horikoshii acylphos- phatase
5
(410) ·
$ 7.99 ·
In stock
Description
A Rigidifying Salt-Bridge Favors the Activity of Thermophilic Enzyme at High Temperatures at the Expense of Low-Temperature Activity

PDF) Crystal Structure of a Hyperthermophilic Archaeal Acylphosphatase from Pyrococcus horikoshii Structural Insights into Enzymatic Catalysis, Thermostability, and Dimerization † , ‡
RCSB PDB - 1W2I: Crystal structuore of acylphosphatase from Pyrococcus horikoshii complexed with formate
A Rigidifying Salt-Bridge Favors the Activity of Thermophilic Enzyme at High Temperatures at the Expense of Low-Temperature Activity
Information on EC 1.13.11.12 - linoleate 13S-lipoxygenase - BRENDA Enzyme Database

Parallel molecular mechanisms for enzyme temperature adaptation

Co-immunoprecipitation of SERCA2a and acylphosphatase (ACP) with

Parallel molecular mechanisms for enzyme temperature adaptation
Comprehensive mutagenesis to identify amino acid residues contributing to the difference in thermostability between two originally thermostable ancestral proteins
Full article: Common patterns and unique features of P-type ATPases: a comparative view on the KdpFABC complex from Escherichia coli (Review)

Crystallographic Data and Refinement Statistics diffraction data
A Rigidifying Salt-Bridge Favors the Activity of Thermophilic Enzyme at High Temperatures at the Expense of Low-Temperature Activity
A rigidifying salt-bridge favors the activity of thermophilic enzyme at high temperatures at the expense of low-temperature activity - Document - Gale OneFile: Health and Medicine

RCSB PDB - 1W2I: Crystal structuore of acylphosphatase from Pyrococcus horikoshii complexed with formate
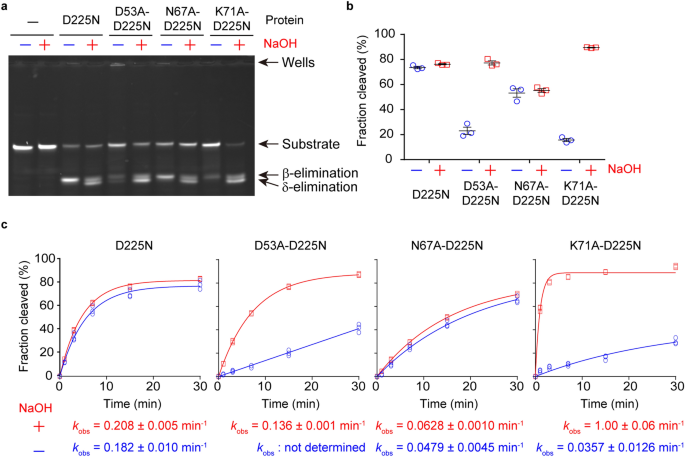
Crystal structure and DNA cleavage mechanism of the restriction DNA glycosylase R.CcoLI from Campylobacter coli
Related products
You may also like
copyright © 2019-2024 richy.com.vn all rights reserved.